Preamble
Calcium Carbonate (CaCO3) is one of the most abundant carbonates in sedimentary rocks across the Earth’s surface. CaCO3 occurs in nature in 6 different forms: three anhydrous crystalline polymorphs.
Calcite, Aragonite, and Vaterite, two hydrated phases and some anhydrous or hydrated amorphous forms. The three polymorphic forms of the crystal show well-differentiated structures
- Calcite (trigonal lattice)
- Aragonite (orthorhombic lattice)
- Vaterite (hexagonal lattice)
as such, the three phases have different diffraction spectra and are therefore easily identifiable with specific investigation methods.
Analysis Methodology
The identification of the different phases of Calcium Carbonate in sanitary water was carried out both by X-ray diffraction analysis (diffractometer) and Infrared Spectroscopy (FT-IR).
Both analyses were conducted on the fixed residue obtained with a specific procedure so as not to affect the analysis itself. The spectra that identify the various forms and crystals of CaCO3 respond with different diffraction angles and therefore provide easily identifiable diagrams (as if each phase had its own fingerprint).
The results obtained from the different samples were compared with the spectra of the pure phases: Calcite – Aragonite – Vaterite.
This comparison was made for both untreated water and water treated with the ExtraH2O device. The analyses were carried out under different conditions: water supply temperature at time zero; temperature 60°C after 36 hours; temperature 80°C at time zero.
Conclusions
In all samples analyzed, the treatment of water with ExtraH2O certainly increases the content of amorphous forms and the presence of possible hydrated forms (highlighted in particular by infrared analyses).
Under these conditions, there is an increase in the solubility of calcium carbonate in water, with a corresponding decrease in the formation of crystals and accumulation of fixed residue.
The disappearance of Calcite and the formation of Aragonite is sharp at temperatures around 60°C. As described in scientific literature, in the analysis conducted after 36 hours at 60°C, Aragonite should have transformed into Calcite (the more stable polymorph), but this transformation did not occur thanks to the water treatment with ExtraH2O.
Upon reaching a temperature of 80°C and analyzing the water at time zero, the system prevents the formation of stable Calcite crystals, leaving only hydrated amorphous forms of various crystals, avoiding precipitation and rapid accumulation of calcareous concretions. According to the literature, the water will tend to reform calcite after a certain period of time. At these temperatures, therefore, the effect of ExtraH2O is temporary.
The innovative PEF technology for the microbial inactivation of food
The present work concerns the application of an innovative system for microbial inactivation, using pulsed electric fields (PEF).
The use of PEFs, documented by analyses of previous experiences and by sector literature suggest that the technique is effective for reducing the bacterial population of liquid foods.
Results
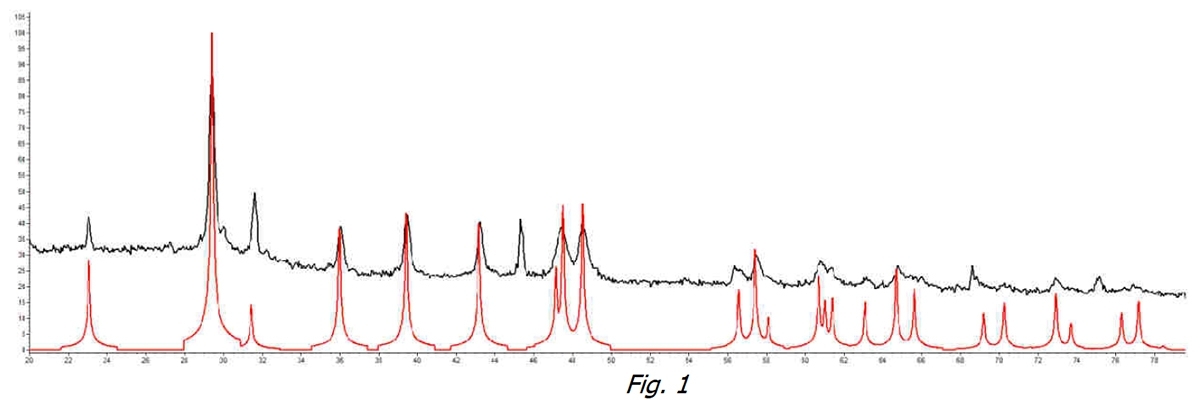
We started by analyzing untreated water. In Figure 1 we can see how and in the untreated water sample at the water supply temperature (black line), there is an identifying peak of Calcite (for comparison with the red line, pure Calcite reference) that identifies this crystalline form as the prevalent one.
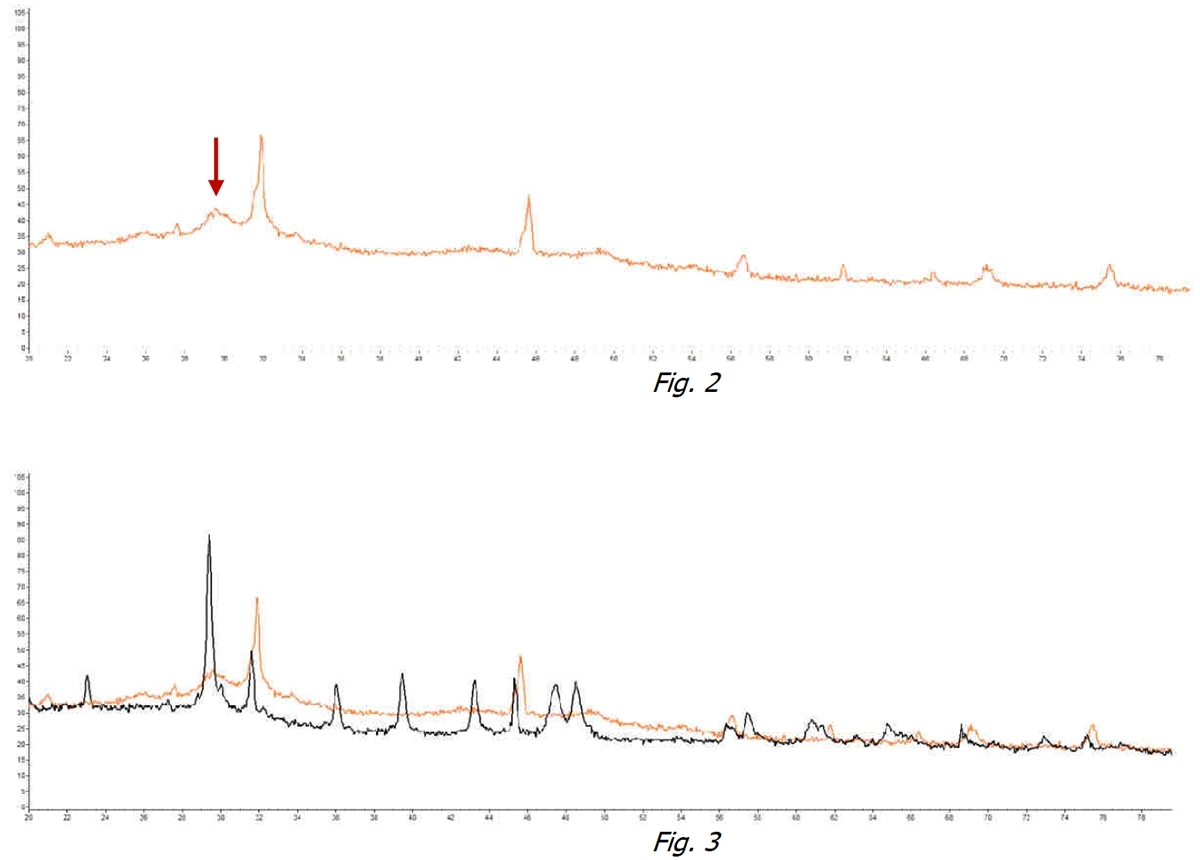
In Figure 2 it can be noticed that in the same water subjected to treatment with the ExtraH2O device, the identifying peak of Calcite disappears in favor of a “hump” devoid of a specific peak. Generally, all the identifying peaks of crystallization disappear. This shows that the Calcite crystals did not form but, on the contrary, there exists a non-crystallized hydrated amorphous form.
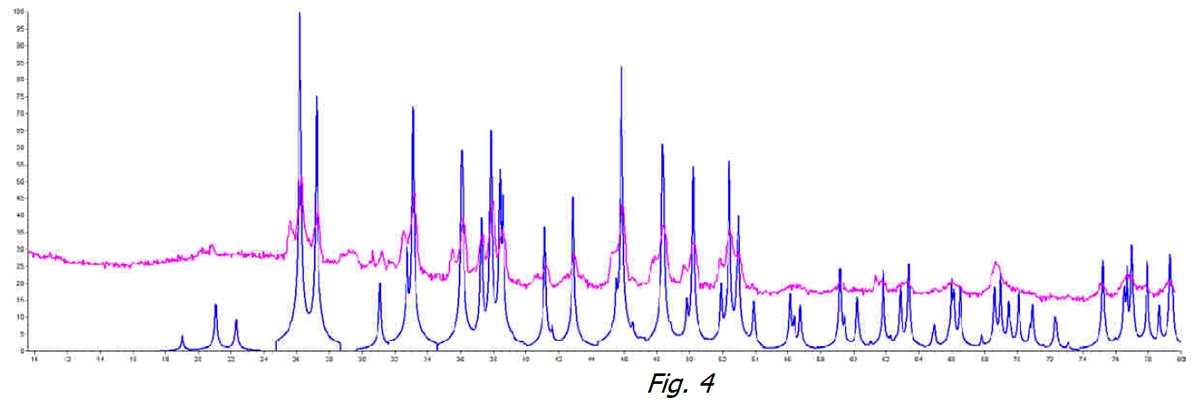
Then, if we compare in Figure 3 the two treated water samples (orange line) and untreated (black line), the phenomenon of non-crystallization is clearly visible. The CaCO3 has no way of aggregating in any of its stable crystalline forms.
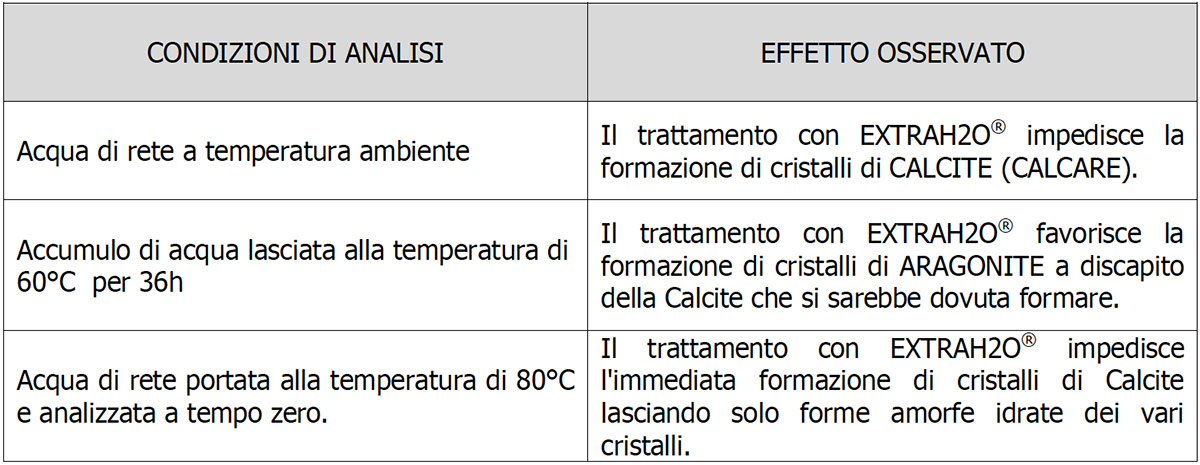
The sample of water treated with ExtraH2O and left for 36 hours at a temperature of 60°C (Fig.4) shows the clear presence of Aragonite in crystalline form. The spectrum of the sample (fuchsia line) perfectly overlaps that of pure Aragonite (blue line). As reported also in literature, at 60°C the most stable phase of CaCO3 is Aragonite, in total absence of crystalline Calcite.
In Figure 5, the untreated sample at room temperature (black line) can be compared to the treated sample brought to 80°C (purple line). The graph clearly shows how all the peaks have been reduced, demonstrating that stable Calcite crystals are unable to form.